A year after the passage of the Inflation Reduction Act (IRA), Medicare negotiation reached its first major milestone, with the federal government and pharmaceutical manufacturers entering into agreements to negotiate what Medicare will pay for the first set of 10 drugs. This development highlighted an important question: What is a drug?
The Food and Drug Administration (FDA) treats a drug as a mixture of active and inactive ingredients. The active ingredient is what gives the drug its therapeutic effect; the specific combination of ingredients is commonly referred to as its “formulation.” Pharmaceutical manufacturers often create different formulations containing the active ingredient. For example, a drug initially marketed as an oral tablet might later be introduced as a cream for patients with a different condition. Manufacturers often introduce new formulations to create a new price for an older product or to guard against competition from a generic version of a drug’s older formulation. Although a generic contains the same active ingredient as older and newer branded formulations, patients can end up switching to a newer branded version. Encouraging patients to move from an older formulation to a newer one is often referred to as product hopping.
Different drug formulations could create confusion for Medicare negotiation: negotiating only some of a drug’s formulations would allow manufacturers to encourage patients to use the versions without negotiated prices. This would increase costs for both patients and Medicare.
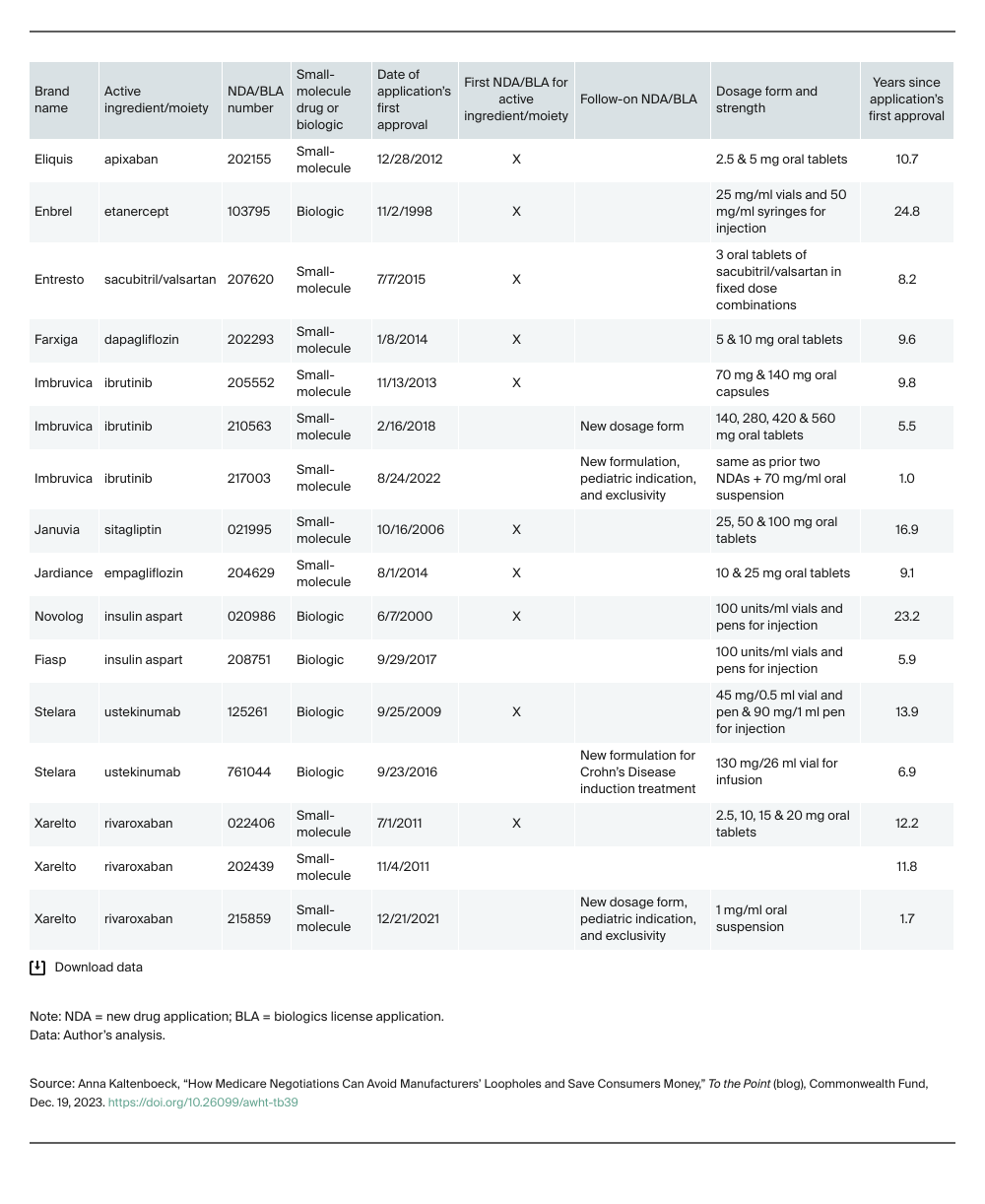
The IRA instructs Medicare to select drugs for negotiation once they’ve been on the market for seven or 11 years for small-molecule drugs and biologics, respectively. The clock starts with FDA approval of a drug’s first application for marketing, known as a new drug application (NDA) for small molecules, and biologics license application (BLA) for biologics. To market additional formulations, manufacturers often add onto the drug’s first application. However, for some formulation changes, like a new inactive ingredient, the FDA requires a new application, even though the active ingredient stays the same. To avoid treating such new formulations as distinct drugs, the IRA instructs Medicare to negotiate all of a drug’s dosage forms and strengths, including new formulations, and to apply the same price across all.
This blog post examines the implications of this approach, looking at the first 10 drugs selected for negotiation.
Looking at Selected Drugs in the First Round of Medicare Negotiations
All 10 selected drugs are available in multiple dosage forms and strengths. Most were available in different formulations, such as oral tablets and suspensions. Four drugs had formulations approved under additional NDAs or BLAs. All but one of these follow-on applications were recent enough that treating their formulations as distinct drugs would exclude them from negotiation, thereby reducing savings.