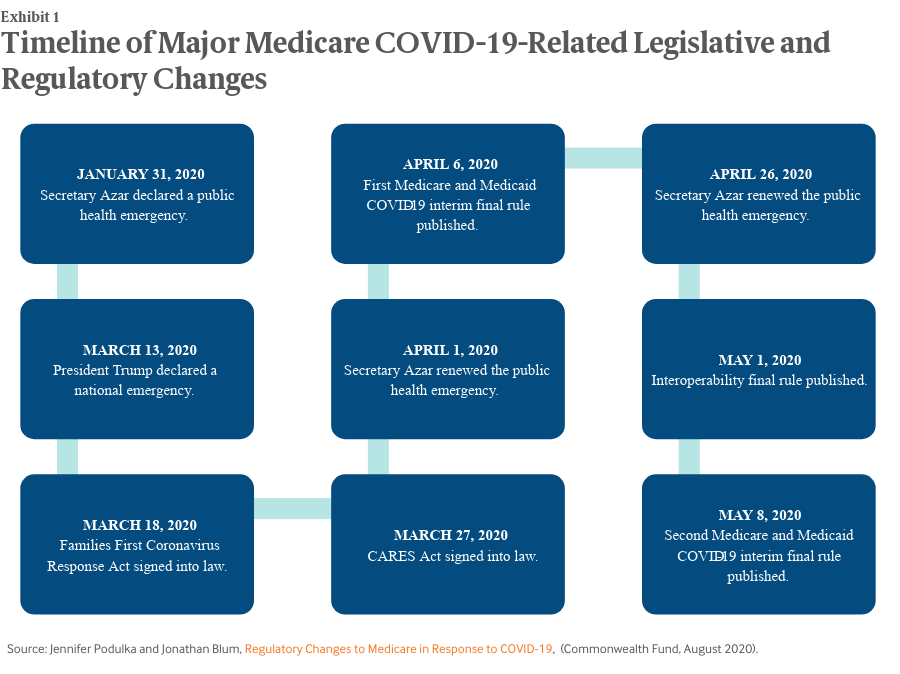
Key Findings
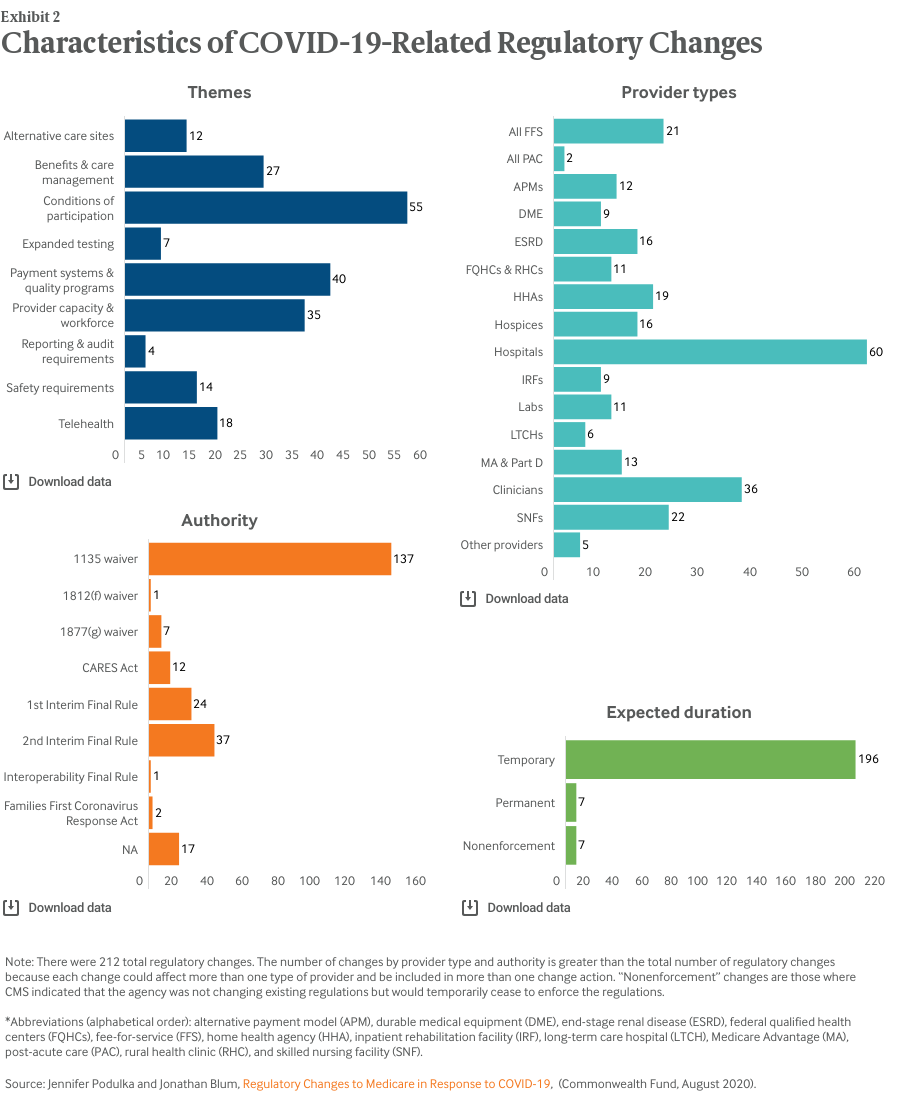
Our analysis found that actions taken by Congress and the administration in response to the COVID-19 pandemic affected virtually all types of health care providers and health plans that participate in the Medicare program (Exhibit 2). To date, efforts have been concentrated on hospital providers, accounting for 60 of the 212 policy actions.
We organized the policies into nine themes. According to our categorization, actions taken by Congress and the administration primarily addressed conditions of participation requirements for Medicare providers (55). The next most common themes were payment systems and quality programs (40) and provider capacity and workforce (35).
Nearly all these policy actions (203) are currently expected to be temporary (Exhibit 2), absent further Congressional or administration action. Seven of the actions were not direct changes to or waivers of regulations; instead CMS indicated that, for a limited time, it would not enforce the existing regulations.
Most policies (145) were implemented through HHS’s various waiver authorities (Exhibit 2). CMS implemented 61 changes through two interim final rules with comment periods. Most, but not all, of the changes included an indication of the effective date and end date.
Most of the changes (179) went into effect sometime during March and many (130) are expected to be in effect through the duration of the public health emergency.8
Discussion
Since the COVID-19-related changes have been in effect for several months, key questions include:
- How were the regulatory changes designed to help the health care system respond to the current public health emergency?
- What are the potential positive and negative impacts of these regulatory changes on Medicare beneficiaries’ access to care?
- What was the purpose of the existing, pre-COVID Medicare regulations?
- If the temporary COVID-19-related regulatory changes become permanent, how can the public have meaningful input on the potential positive and negative consequences?
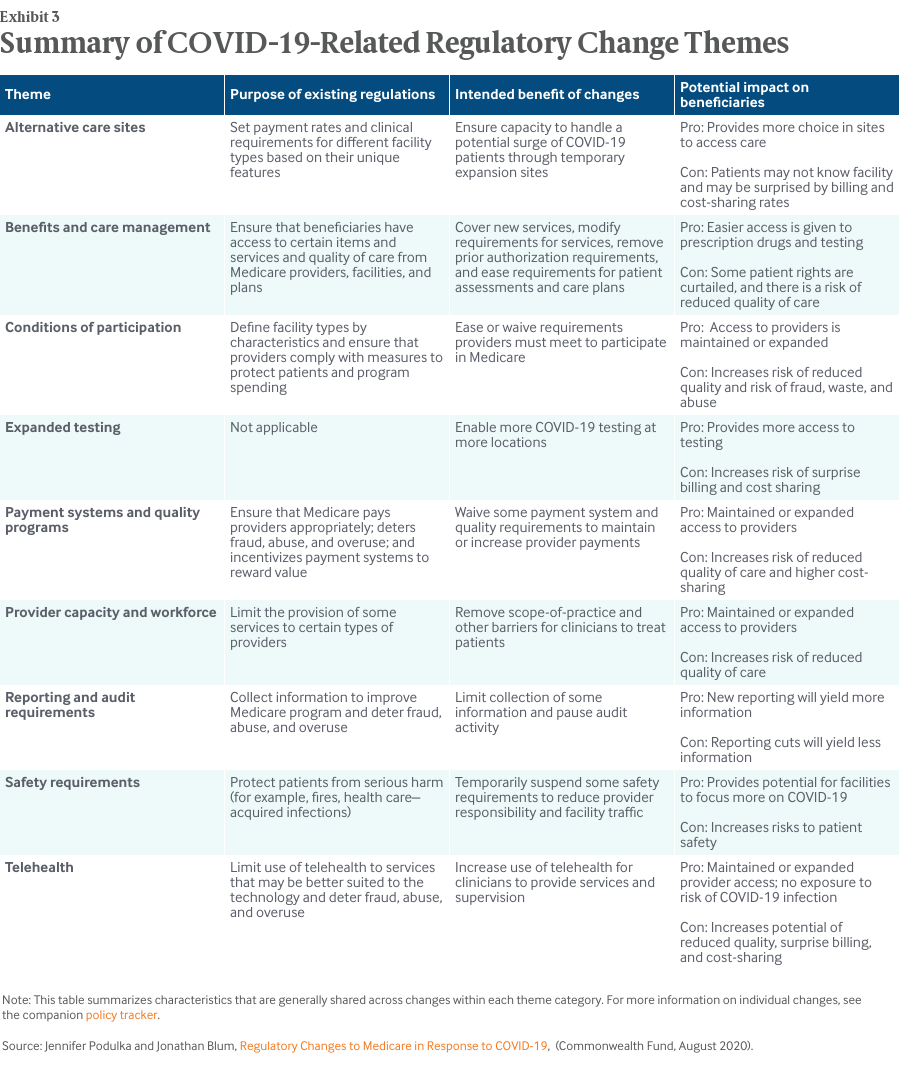
The nine themes that HMA assigned to each of the regulatory changes help to address the first question. In general, the regulatory changes were designed to assist health care systems in testing and treating a surge of COVID-19 patients and in enabling health care providers to continue treating Medicare patients under social distancing requirements. With more than 200 individual regulatory changes, there is a wide range of specific intended benefits (Exhibit 3).
Most changes to Medicare regulations bring both potential positive and negative impacts for Medicare beneficiaries and the health care systems that provide care to them. In response to the second question, Exhibit 3 highlights some potential positive and negative impacts for Medicare beneficiaries. For example, even highly necessary and appropriate changes, like increased COVID-19 testing, come with potential drawbacks for beneficiaries, such as the risk of surprise billing and high cost-sharing amounts.
As to the third question, Exhibit 3 indicates that existing Medicare regulations that have been temporarily or permanently waived by COVID-19-related changes fulfill critical public policy purposes for the program, such as:
- ensuring that beneficiaries have access to certain items and services
- ensuring that providers comply with measures to protect patients
- deterring fraud, abuse, and overuse
- protecting patients from serious harm.
It is also important to note that Medicare regulations, under normal circumstances, go into effect only after going through an established notice-and-comment rulemaking process, which enables stakeholders to raise questions and provide input that the agency can use to determine the best course of action. HHS and CMS may waive or modify this process if they can demonstrate good cause, such as public emergencies.
While the majority of COVID-19-related regulatory changes were declared to be temporary when they were announced, the administration has indicated plans to make some permanent.9 Changes that have proved popular with providers and patients are likely candidates, such as expanded reimbursement for telemedicine services. Stakeholders have expressed eagerness to learn which changes may become permanent, and the administration has taken actions to gather input on these decisions. On June 23, 2020, CMS announced the creation of a new division in the agency, the Office of Burden Reduction and Health Informatics, which is tasked with continuing “to explore innovative ways to address regulatory reform and burden reduction.”10
It may be necessary to let certain temporary waivers expire, possibly even before conclusion of the public health emergency if the continued threat of possible patient harm outweighs the potential benefits of the policy waiver, such as reducing the overall number of people coming through health care facilities. For example, policymakers may need to significantly limit the duration of the waivers related to on-time preventive maintenance of dialysis machines and scheduled fire inspections.
To answer the fourth question, it will be essential to make use of the unique opportunity presented by the temporary policy changes to study their impact, especially any unintended consequences. These analyses should compare the trade-offs in positive and negative consequences for beneficiaries (including populations such as dual-eligibles), providers, and taxpayers. The analytic results, along with stakeholder comments, should be part of regular notice-and-comment rulemaking to determine which regulatory changes should be made permanent.
Conclusion
In response to the COVID-19 public health emergency, Congress and the Trump administration made an unprecedented number of legislative, regulatory, and subregulatory changes to the Medicare program. While these policy actions were designed to address urgent concerns — protecting beneficiaries’ access to care through use of alternative care settings and Medicare-reimbursed telemedicine services, as well as supporting provider and health plan responses through temporary relief from certain requirements — the actions come with the risk of negative outcomes. Those risks include trading the important, long-standing beneficiary protections and Medicare spending controls included in the original regulations for policies that may, on balance, prove less preferable.
As the COVID-19 pandemic continues, policymakers and stakeholders should carefully assess both the benefits and unintended consequences of these policy actions for patients’ access to care and the ability of providers to provide high-quality care. The analyses and stakeholder input should inform the regular notice-and-comment rulemaking process to ensure that any permanent regulatory changes improve the Medicare program. In addition, the effects of these policies should be carefully studied to help determine the best way to prepare for future public health emergencies.